BtaEX0023431 @ bosTau6
Exon Skipping
Gene
ENSBTAG00000015988 | MYH11
Description
myosin-11 [Source:RefSeq peptide;Acc:NP_001095597]
Coordinates
chr25:14280607-14284576:-
Coord C1 exon
chr25:14284474-14284576
Coord A exon
chr25:14282902-14282922
Coord C2 exon
chr25:14280607-14280699
Length
21 bp
Sequences
Splice sites
3' ss Seq
ACTGTATTGTCCAACCACAGCAA
3' ss Score
5.03
5' ss Seq
TACGTGAGT
5' ss Score
7.77
Exon sequences
Seq C1 exon
AGGCGAATCTGGAGCCGGGAAAACAGAAAACACAAAGAAAGTCATCCAGTACCTGGCCATGGTGGCCTCCTCCCACAAAGGCAAGAAGGATACGAGCATCACG
Seq A exon
CAAGGCCCATCTTTTGCCTAC
Seq C2 exon
GGTGAGCTGGAGAAGCAGCTTCTACAAGCAAACCCCATCCTGGAGGCCTTTGGCAACGCCAAAACGGTCAAGAACGACAACTCCTCCCGATTC
VastDB Features
Vast-tools module Information
Secondary ID
ENSBTAG00000015988_CASSETTE3
Average complexity
S
Mappability confidence:
100%=100=100%
Protein Impact
Alternative protein isoforms (No Ref)
No structure available
Features
Disorder rate (Iupred):
C1=0.114 A=0.000 C2=0.000
Domain overlap (PFAM):
C1:
PF0006316=Myosin_head=FE(5.0=100)
A:
PF0006316=Myosin_head=FE(0.9=100)
C2:
PF0006316=Myosin_head=FE(4.4=100)
Main Inclusion Isoform:
ENSBTAT00000035455fB4518
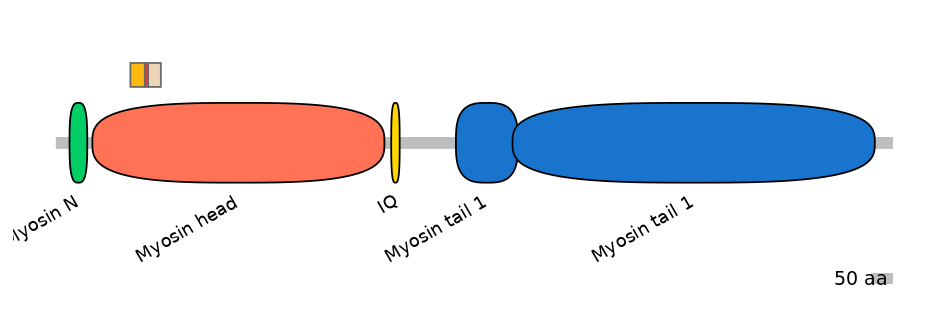
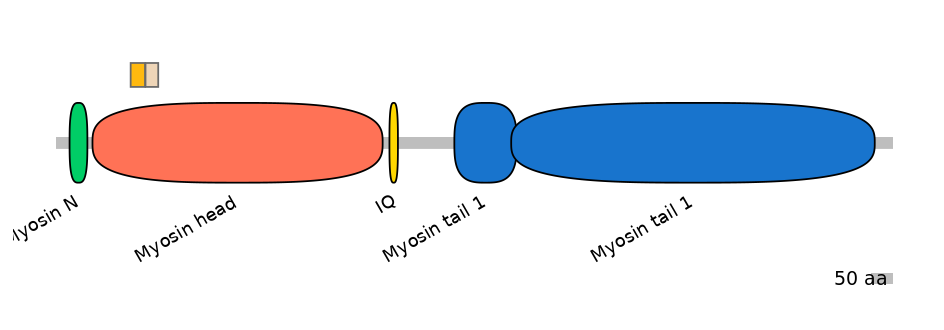
Other Inclusion Isoforms:
NA
Other Skipping Isoforms:
NA
Associated events
Conservation
Primers PCR
Suggestions for RT-PCR validation
F:
CCTCCCACAAAGGCAAGAAGG
R:
GTCGTTCTTGACCGTTTTGGC
Band lengths:
113-134
Functional annotations
There are 10 annotated functions for this event
PMID: 8509418
Chicken gizzard myosin, that is almost exclusively comprised of the (+)insert iso- form, produces a 2.5-fold higher actin filament velocity in an in vitro motility assay and a 2.0-fold higher myosin ATPase activity than myosin isolated from bovine aorta, which expresses little (+)insert. Importantly, these results in the motility assay were the same after LC17a/LC17b exchange, that is when both gizzard and aorta myosins had the same light chain composition. This suggested that the difference in velocity is due to the heavy chain itself and not LC17.
PMID: 9147986
A baculovirus expression system was used to construct hybrid heavy meromyosins (HMM) as an alternative way to explore the function of this microexon. When the insert was removed, the velocity of chicken gizzard HMM in the in vitro motility assay was reduced by two-fold and the actin-activated myosin ATPase activity was reduced 2.2-fold. Conversely, the velocity and myosin ATPase rates were increased by two-fold when the insert was added into uterine HMM. These results led to the important conclusion that the insert is both necessary and sufficient to explain the two-fold greater rate of actin filament movement and actin-activated myosin ATPase activity produced by the (+)insert SMMHC isoform.
PMID: 9887959
A laser trap was used to characterize the molecular mechanics of the inserted isoform [(+)insert] and of a mutant lacking the insert [(-)insert], which is analogous to the isoform found in tonic muscles. The constructs were expressed in the baculovirus/insect cell system. Unitary displacements (Duni) were similar for both the constructs (approximately 10 nm) but the attachment time (ton) for the (-)insert was two times that of the (+)insert. These data suggest that the insert in the nucleotide-binding loop does not affect the inherent mechanics of the myosin molecule but rather the kinetics of the cross-bridge cycle.
PMID: 10047983
Laser trap was used to characterize the molecular mechanics and kinetics of the inserted isoform ((+)insert) and of a mutant lacking the insert ((-)insert), analogous to the isoform found in tonic muscle. The constructs were expressed as heavy meromyosin using the baculovirus/insect cell system. Unitary displacement (d) was similar for both constructs (approximately 10 nm) but the attachment time (t(on) for the (-)insert was twice as long as for the (+)insert regardless of the [MgATP]. Both the relative average isometric force (Favg(-insert)/Favg(+insert) = 1.1 +/- 0.2 (mean +/- SE) using the in vitro motility mixture assay, and the unitary force (F approximately 1 pN) using the laser trap, showed no difference between the two constructs. However, as under unloaded conditions, t(on) under loaded conditions was longer for the (-)insert compared with the (+)insert construct at limiting [MgATP]. These data suggest that the insert in this surface loop does not affect the mechanics but rather the kinetics of the cross-bridge cycle.
PMID: 16716643
[Review]. Microexon encoding seven amino acids [(+)insert] or absence [(-)insert] in the motor domain regulates contractive properties of myosin heavy chain. This is a comprehensive review on the expression and biological functions of the isoforms. In particular, it discusses a large number of studies correlating isoform expression and ATPase activity and rate of tissue shortening in smooth muscle (in multiple WT tissues and in disease contexts).
PMID: 11715025
Loss of SM-B myosin in an microexon-specific mouse KO does not affect survival or cause any overt smooth muscle pathology. Physiological analysis reveals that absence of SM-B myosin results in a significant decrease in maximal force generation and velocity of shortening in smooth muscle tissues. Bladder from the knockout ani- mals demonstrated a 3.1-fold reduction in shortening velocity compared to the wild-type mice whereas the resistance mesenteric blood vessels showed a 1.7-fold reduction. An unpredicted result, however, was the 1.6- fold reduction in maximal tension in the knockout mouse bladder. Thus, the extra seven-residue insert in the surface loop 1 of SM-B myosin is a critical determinant of crossbridge cycling and velocity of shortening.
PMID: 12562924
Exogenous ADP increased force and stiffness in a manner consistent with increasing the Ca2+ concentration in both the +/+ and +/- mouse types. However, the -/- type showed a significantly greater increase in force than in stiffness suggesting that immediately prior to ADP release, the AMATPase either has an additional force producing isomerization state or a slower ADP dissociation rate for the -/- type compared to the +/+ or +/- types. Exogenous Pi led to a significantly greater decrease in stiffness than in force for all three mouse types suggesting that there is a force producing state prior to Pi release. In addition, the increase in Pi showed similar changes in the +/+ and -/- types whereas in the +/- type the decreases in both force and stiffness were greater than the other two mouse types indicating that the insert can affect the cooperativity between myosin heads.
PMID: 12959948
This study measured the time course of bronchoconstriction following a bolus injection of methacholine (160 microg/kg) in (+)insert isoform knockout (KO) and corresponding wild-type (WT) mice. Neither baseline airway resistance (Raw) (0.424 +/- 0.04 for WT and 0.374 +/- 0.01 cm H(2)O.s.ml(-1) for KO) nor peak Raw (4.1 +/- 0.9 for WT and 4.0 +/- 0.5 cm H(2)O.s.ml(-1) for KO) differed between groups. However, the time to peak Raw was significantly longer in the KO (17.2 +/- 0.6 s) compared with the WT (14.6 +/- 0.8 s) mice (P < 0.05). Differentiating Raw with respect to time revealed a greater rate of bronchoconstriction for the WT during the initial 4 s, presumably reflecting the faster shortening velocities under these relatively unloaded conditions. Reverse transcriptase-polymerase chain reaction analysis revealed that the (+)insert myosin isoform mRNA content in the WT airways was 47.8 +/- 5.6%.
PMID: 15140746
Contrary to bladder, maximal force was increased by _50% in the microexon knockout mesenteric blood vessels. Reduced velocity (from 15% to 50%) and increased force (19% with KCl and 37% with phenylephrine) were also observed for aorta. This was surprising given that the aorta has little or no (+)insert in wild-type animals. However, additional structural alterations were observed in aorta, including a two-fold increased expression of LC17b, and a 1.4-fold increase for calponin, whereas caldesmon expression was decreased by _50%. A decrease in caldesmon expression of about 30% was also observed in bladder.
PMID: 16316347
[Negative results]. Constriction amplitudes, dc/dt(max), and t(1/2) of afferent as well as efferent arterioles upon Ang II stimulation were similar in smb(+/+) and smb(-/-) mice. Constriction amplitudes upon KCl stimulation of afferent arterioles were similar in both smb(+/+) and smb(-/-) mice. Furthermore, KCl-induced dc/dt(max) and t(1/2) of afferent arterioles were similar in both smb(+/+) and smb(-/-) mice. SMB expression could be detected in afferent but not efferent arterioles in smb(+/+) mice. No SMB expression in either arteriole could be observed in smb(-/-) mice.
GENOMIC CONTEXT[edit]
INCLUSION PATTERN[edit]
SPECIAL DATASETS
- Pre-implantation embryo development