DreEX0033173 @ danRer10
Exon Skipping
Gene
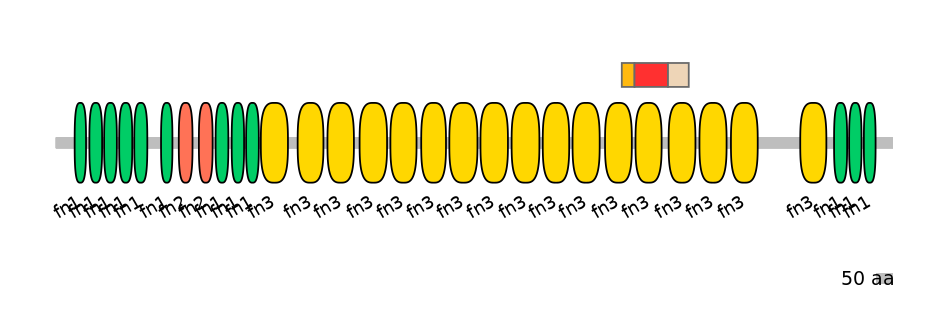
ENSDARG00000006526 | fn1b
Description
fibronectin 1b [Source:ZFIN;Acc:ZDB-GENE-030131-6545]
Coordinates
chr1:4814629-4815554:-
Coord C1 exon
chr1:4815441-4815554
Coord A exon
chr1:4814920-4815219
Coord C2 exon
chr1:4814629-4814816
Length
300 bp
Sequences
Splice sites
3' ss Seq
TACTTTGTCCTTCTTTCTAGCTA
3' ss Score
9.07
5' ss Seq
TTGGTATGG
5' ss Score
5.53
Exon sequences
Seq C1 exon
AGCGTACAGAAATGACCATCCATGGCTTGGTGCCCACGGTGGAGTATACAATCAATGTGGTTGCTCTCAGCCAAGAAGGAGAAAGCACACCAGTGGTCCAAAAAGCTACAACAA
Seq A exon
CTAATGAACAGAAACCAAAAGACCTGACCTTCACTGATGTGGACTCTACCTCCATGCTTGTGAAATGGAATGTTCCACGGGTTCCAGGAGTGACCTCCTACAGGGTTCTGTATTCTAGTCCGGAGGAAGGCGAAAGAGAGTACCAACCTGCCTTAAGTGGACGTGATAACAGTGTGCTTCTTCAAGGTCTGAACCCCGGAACCATGTACATCATTAAAGTTATTCCAATGAAAGGACGCAACCCACTTCAAACCCTTGAGGGCATACAGCAAACTATCCAAGAAGACACTCAGCGCATTG
Seq C2 exon
CTGTTCCTGCTCCCACCGATATTCAGTTCTTCGACATCAGTCCGTCTTCCTTTATTGTGTCATGGCAAGCACCCAATTCCAGACTTTCTGGCTATCGTGTGGTGGTCACCCCAAGGAATCAGCTTGCCACATCCAAAGAAATGACCATTTCACCAGACTCTACGCAAGTCTTAGTGCCTGGGCTTGTG
VastDB Features
Vast-tools module Information
Secondary ID
ENSDARG00000006526_MULTIEX1-9/9=8-C2
Average complexity
S
Mappability confidence:
100%=100=100%
Protein Impact
Alternative protein isoforms (Ref)
No structure available
Features
Disorder rate (Iupred):
C1=0.538 A=0.396 C2=0.111
Domain overlap (PFAM):
C1:
PF0004116=fn3=PD(37.0=76.9)
A:
PF0004116=fn3=WD(100=78.2)
C2:
PF0004116=fn3=PU(74.1=95.2)
Main Skipping Isoform:
NA
Other Skipping Isoforms:
NA
Associated events
Conservation
Rat
(rn6)
No conservation detected
Fruitfly
(dm6)
No conservation detected
Primers PCR
Suggestions for RT-PCR validation
F:
AGCGTACAGAAATGACCATCCA
R:
CACAAGCCCAGGCACTAAGAC
Band lengths:
302-602
Functional annotations
There are 16 annotated functions for this event
PMID: 7806580
The study examined the cell-specific expression of two fibronectin isoforms, EIIIA (contains MmuEX0019422) and EIIIB (contains MmuEX0019423), during experimental hepatic fibrosis induced by ligation of the biliary duct. AT the mRNA level, EIIIA and EIIIB were undetectable in normal liver but expressed early injury, preceding fibrosis. The cellular sources of these changes were determined by fractionating the liver at various time points after bile duct ligation into its constituent cell populations and extracting RNA from the fresh isolates. EIIIA-containing fibronectin mRNA was undetectable in normal sinusoidal endothelial cells but increased rapidly within 12 h of injury. By contrast, the EIIIB form was restricted to hepatic lipocytes (Ito or fat-storing cells) and appeared only after a lag of 12-24 h: it was minimal in sinusoidal endothelial cells. Both forms were minimal in hepatocytes. At the protein level, EIIIA-containing fibronectin was markedly increased within two days of injury and exhibited a sinusoidal distribution. Secretion of this form by endothelial cells was confirmed in primary culture. Matrices deposited in situ by endothelial cells from injured liver accelerated the conversion (activation) of normal lipocytes to myofibroblast-like cells, and pretreatment of matrices with monoclonal antibody to the EIIIA segment blocked this response. Finally, recombinant fibronectin peptide containing the EIIIA segment was stimulatory to lipocytes in culture.
PMID: 12847088
To study the function of extra domain A (EDA) (contains MmuEX0019422) of fibronectin 1 (FN) in vivo, the authors generated mice devoid of EDA exon-regulated splicing. Constitutive exon inclusion was obtained by optimizing the splice sites, whereas complete exclusion was obtained after in vivo CRE-loxP-mediated deletion of the exon. Homozygous mouse strains with complete exclusion or inclusion of the EDA exon were viable and developed normally, indicating that the alternative splicing at the EDA exon is not necessary during embryonic development. Conversely, mice without the EDA exon in the FN protein displayed abnormal skin wound healing, whereas mice having constitutive inclusion of the EDA exon showed a major decrease in the FN levels in all tissues. Moreover, both mutant mouse strains have a significantly shorter lifespan than the control mice, suggesting that EDA splicing regulation is necessary for efficient long-term maintenance of biological functions.
PMID: 14976060
In atherosclerotic lesions, fibronectin 1 (FN) including the EIIIA domain (EIIIA-FN) (also called EDA)(MmuEX0019422), is abundant, whereas FN in the flanking vessel wall lacks EIIIA. Lesional EIIIA-FN is localized with endothelial cells and macrophage foam cells. To directly test the function of EIIIA-FN, the study generated EIIIA-null (EIIIA(-/-)) mice that lack the EIIIA exon and crossed them with apolipoprotein E (ApoE)-null (ApoE(-/-)) mice that develop arterial wall lesions. Compared with ApoE(-/-) controls, EIIIA(-/-)ApoE(-/-) mice had significantly smaller lesions throughout the aortic tree. EIIIA-FN was increased in ApoE(-/-) plasma, and total plasma cholesterol was reduced in EIIIA(-/-)ApoE(-/-) mice, specifically in large lipoprotein particles, suggesting a functional role for plasma EIIIA-FN. To assess a role for macrophage EIIIA-FN in the vessel wall, in vitro foam cell assays were conducted. EIIIA(-/-)ApoE(-/-) macrophages accumulated significantly less intracellular lipid than control ApoE(-/-) cells. These results provide genetic evidence that suggests roles for EIIIA-FN in plasma lipoprotein metabolism and in foam cell formation.
PMID: 15367684
[Negative results]. Fibronectin 1 (FN) splice variants containing the EIIIA (MmuEX0019422) and/or EIIIB (MmuEX0019423) exons are prominently expressed in the vasculature of a variety of human tumors but not in normal adult tissues. To understand the functions of these splice variants in physiological and tumor angiogenesis, thus study used EIIIB-null and EIIIA-null strains of mice to examine neovascularization of mouse retinas, pancreatic tumors in Rip-Tag transgenic mice, and transplanted melanomas. Contrary to expectations, physiological and tumor angiogenesis was not significantly affected by the absence of either EIIIA or EIIIB splice variants. Tumor growth was also not affected. In addition, the expression levels of smooth muscle alpha actin, believed to be modulated by EIIIA-containing fibronectins, were not affected either.
PMID: 15610531
The fibronectins (FN) comprise a family of adhesive extracellular matrix proteins thought to mediate important functions in cutaneous wounds. Plasma fibronectin (pFN) extravasates for days from intact hyperpermeable vessels following injury whereas mRNAs encoding the cellular fibronectins (cFN) that include two segments, termed EIIIA (EDA) (MmuEX0019422) and EIIIB (EDB) (MmuEX0019423), are expressed by wound cells. Wounds in mice null for pFN appear to heal normally whereas those in EIIIA null mice exhibit defects, suggesting that cFN may play a role when pFN is missing. Integrin alpha9beta1, a receptor for several extracellular matrix proteins as well as the EIIIA segment, is expressed normally in the basal layer of squamous epithelia. The study reports results from immunohistochemistry on healing wounds demonstrating that EIIIA-containing cFN are deposited abundantly but transiently from day 4 to 7 whereas EIIIB-containing cFN persist at least through day 14. Elevated expression of alpha9beta1 is seen in basal and suprabasal epidermal keratinocytes in wounds. The spatial expression patterns of cFN and alpha9beta1 are distinct, but overlap in the dermal-epidermal junction, and both are expressed contemporaneously. These observations suggest a role for alpha9beta1-EIIIA interactions in wound keratinocyte function.
PMID: 17202335
Vaccination strategies based on the in vivo targeting of antigens (Ags) to dendritic cells (DCs) are needed to improve the induction of specific T cell immunity against tumors and infectious agents. In this study, the authors used a recombinant protein encompassing the extra domain A from fibronectin (EDA) (MmuEX0019422), an endogenous ligand for TLR4, to deliver Ags to TLR4-expressing DC. The purified EDA protein was shown to bind to TLR4-expressing HEK293 cells and to activate the TLR4 signaling pathway. EDA also stimulated the production by DC of proinflammatory cytokines such as IL-12 or TNF-alpha and induced their maturation in vitro and in vivo. A fusion protein between EDA and a cytotoxic T cell epitope from ovalbumin (OVA) efficiently presented this epitope to specific T cells and induced the in vivo activation of a strong and specific cytotoxic T cell (CTL) response. Moreover, a fusion protein containing EDA and the full OVA also improved OVA presentation by DC and induced CTL responses in vivo. These EDA recombinant proteins protected mice from a challenge with tumor cells expressing OVA. These results strongly suggest that the fibronectin extra domain A may serve as a suitable Ag carrier for the development of antiviral or antitumoral vaccines.
PMID: 17575266
The activation of mast cells by extra domain A of fibronectin (FN-EDA) (contains HsaEX0026101), an endogenous ligand of TLR4, and its contribution to the pathogenesis of rheumatoid arthritis (RA) in vivo were examined. FN-EDA, but no other domain of the fibronectin fragment, III(11) (FN-III(11)) and III(12) (FN-III(12)), stimulated bone marrow-derived murine mast cells (BMMCs) dose-dependently to secret cytokines (TNF-alpha, IL-6, and IL-1beta), similar to the pattern produced by LPS. FN-EDA-induced cytokine production was mediated by TLR4, as cytokine production by FN-EDA was absent in TLR4-deficient (TLR4-/-) BMMCs. The study also examined the roles of TLR4-mediated mast cell activation by this form of fibronectin fragment in the pathogenesis of RA in vivo. The injection of FN-EDA, but not FN-III(11)and FN-III(12), to joints resulted in joint swelling of mice in vivo. Genetically mast cell-deficient WBB6F(1)-W/W(v) mice exhibited significantly less swelling and cytokine production compared with mast cell-sufficient +/+ mice, suggesting that swelling and inflammatory cytokine production were partially dependent on tissue mast cells. Reduced swelling and cytokine production were recovered by the reconstitution of tissue mast cells by the injection of BMMCs from wild-type mice but not from TLR4-/- mice. Altogether, these results suggest that the TLR4-mediated activation of mast cells by endogenous ligand FN-EDA might contribute to the pathogenesis of RA through proinflammatory cytokine production.
PMID: 17644525
Mice constitutively expressing extra domain A-containing FN (EDA(+)FN) (contains MmuEX0019422) have a significant decrease of FN levels in plasma and most tissues. Hepatocytes modified to produce EDA(+)FN have normal extracellular matrix-FN levels but secrete less soluble FN. In a liver-specific EDA-exon deletion in these animals, FN levels were restored both in plasma and tissues. Therefore, an important fraction of tissue FN, approximately an equal amount of that produced by the tissue itself, is actually plasma-derived, suggesting that plasma is an important source of tissue FN.
PMID: 17706958
Alternatively spliced variants of fibronectin (FN) containing exons EIIIA (MmuEX0019422) and EIIIB (MmuEX0019423) are expressed around newly forming vessels in development and disease but are downregulated in mature vasculature. The sequences and patterns of expression of these splice variants are highly conserved among vertebrates, suggestive of their biological importance; however the functions of EIIIA and EIIIB-containing FNs are unknown. To understand the role(s) of these splice variants, the authors deleted both EIIIA and EIIIB exons from the FN gene and observed embryonic lethality with incomplete penetrance by embryonic day 10.5. Deletion of both EIIIA and EIIIB exons did not affect synthesis or cell surface deposition of FN, indicating that embryonic lethality was due specifically to the absence of EIIIA and EIIIB exons from FN. EIIIA/EIIIB double-null embryos displayed multiple embryonic cardiovascular defects, including vascular hemorrhage, failure of remodeling embryonic and yolk sac vasculature, defective placental angiogenesis and heart defects. In addition, they observed defects in coverage and association with dorsal aortae of alpha-smooth-muscle-actin-positive cells. These studies indicate that the presence or absence of EIIIA and EIIIB exons alters the function of FN and demonstrate the requirement for these alternatively spliced exons in cardiovascular development.
PMID: 17897651
To analyze the role of fibronectin (FN) isoforms (with/without extra domain A (EDA) (MmuEX0019422)) in atherosclerotic lesion formation the authors utilized mouse strains devoid of EDA exon regulated splicing, which constitutively include (EDA(+/+)) or exclude (EDA(-/-)) the exon. Both mutant mice had a 40% reduction in atherosclerotic lesions after the atherogenic-diet treatment (mean+/-S.E., microm(2); 22969+/-2185; 13660+/-1533; 14260+/-2501 for EDA(wt/wt), EDA(+/+) and EDA(-/-), respectively; p< or =0.01 ANOVA test) associated to a lower capacity of macrophages to uptake modified LDL and undergo foam-cell formation. Lesions in control mice were more numerous and bigger, with augmented and deeper macrophage infiltration, and increased FN expression in the sub-endothelial area. Previous experiments have shown that apoE(-/-)EDA(-/-) mice have a decreased number and size of atherosclerotic lesions and, on this basis, it has been proposed that the EDA domain has a pro-atherogenic role. These data with the EDA(+/+) mice rules out this hypothesis and suggest that regulated splicing of the EDA exon of the FN gene is involved in progression of atherosclerosis, highlighting the importance of alternative splicing in regulating cellular processes.
PMID: 17991876
Fibronectin (FN) plays an important role in the formation of stable arterial thrombi at the site of vascular injury. FN containing Extra Domain A (EDA+ FN) (MmuEX0019422) is absent from normal plasma, but elevated plasma levels of EDA+ FN are found in several pathological conditions. The authors hypothesized that EDA+ FN plays a special role in thrombosis. They used mouse strains constitutively including (EDA+/+) or excluding (EDA-/-) the EDA domain in all tissues and plasma. Using a flow chamber and the ferric-chloride injury model the authors found that EDA+ FN accelerates thrombosis both in vitro and in vivo at arterial shear rates. In EDA+/+ mice thrombi (>30 microm) grew faster when compared with EDA(WT/WT) (6.6+/-0.2 minutes versus 8.3+/-0.6 minutes, P
PMID: 18096707
Idiopathic pulmonary fibrosis lung fibroblasts produced markedly more extra domain A (EDA)-containing fibronectin (containing MmuEX0019422) and alpha-smooth muscle actin (alpha-SMA) than control fibroblasts. EDA-null mice failed to develop significant fibrosis 21 days after bleomycin challenge, whereas wild-type controls developed the expected increase in total lung collagen. Histologic analysis of EDA-null lungs after bleomycin showed less collagen and fewer alpha-SMA-expressing myofibroblasts compared with that observed in wild-type mice. Failure to develop lung fibrosis in EDA-null mice correlated with diminished activation of latent transforming growth factor (TGF)-beta and decreased lung fibroblast responsiveness to active TGF-beta in vitro. The data show that EDA-containing fibronectin is essential for the fibrotic resolution of lung injury through TGF-beta activation and responsiveness, and suggest that EDA-containing fibronectin plays a critical role in tissue fibrogenesis.
PMID: 20643910
Fibronectin (FN) is a major component of the extracellular matrix and occurs in two main forms: plasma and cellular FN. The latter includes the alternatively spliced domain A (EDA) (MmuEX0019422). Although EDA-containing cellular fibronectin (EDA-FN) is associated with fibroblast differentiation, how EDA-FN promotes differentiation is incompletely understood. This study investigated the mechanism by which EDA-FN contributes to fibroblast differentiation with emphasis on the characterization of the EDA-FN receptor. EDA-FN increases a-SMA expression (immunofluorescence), collagen deposition, cell contractility, and focal adhesion kinase (FAK) activation (immunoblotting); whereas plasma FN, a form lacking EDA, shows no effect. Primary lung fibroblasts constitutively express a(4)beta(7) integrin receptor (FACS and RT-PCR). Blocking of a(4)beta(7) reduces fibroblast adhesion to EDA-FN and inhibits a-SMA expression, collagen deposition, and FAK activation induced by EDA-FN. Using recombinant EDA-containing peptides, it demonstrated that the EDA segment is sufficient to induce fibroblast differentiation via binding to a(4)beta(7). EDA-FN induces MAPK-Erk1/2 activation and inhibition of MEK1/2 attenuates EDA-FN-induced a-SMA expression. These findings demonstrate that EDA-FN induces fibroblast differentiation by a mechanism that involves binding of EDA to a(4)beta(7) integrin followed by activation of FAK and MAPK-associated signaling pathways
PMID: 21350212
Wild-type mice and mice lacking the extra domain A (EDA) (MmuEX0019422) of fibronectin (fn), referred to as EDA(-/-), underwent permanent ligation of the left anterior coronary artery. Despite equal infarct size between groups (38.2?4.6% versus 38.2?2.9% of left ventricle; P=0.985), EDA(-/-) mice exhibited less left ventricular dilatation and enhanced systolic performance compared with wild-type mice as assessed by serial cardiac MRI measurements. In addition, EDA(-/-) mice exhibited reduced fibrosis of the remote area without affecting collagen production, cross-linking, and deposition in the infarct area. Subsequently, ventricular contractility and relaxation was preserved in EDA(-/-). At tissue level, EDA(-/-) mice showed reduced inflammation, metalloproteinase 2 and 9 activity, and myofibroblast transdifferentiation. Bone marrow transplantation experiments revealed that myocardium-induced EDA and not EDA from circulating cells regulates postinfarct remodeling. Finally, the absence of EDA reduced monocyte recruitment as well as monocytic Toll-like receptor 2 and CD49d expression after infarction. This study demonstrated that parenchymal fn-EDA plays a critical role in adverse cardiac remodeling after infarction. Absence of fn-EDA enhances survival and cardiac performance by modulating matrix turnover and inflammation via leukocytes and fibroblasts after infarction.
PMID: 28325836
Fibronectin is a multidomain protein secreted by various cell types. It forms a network of fibers within the extracellular matrix and impacts intracellular processes by binding to various molecules, primarily integrin receptors on the cells. Both the presence of several isoforms and the ability of the various domains and isoforms to bind to a variety of integrins result in a wide range of effects. In vivo findings suggest that fibronectin isoforms produced by the osteoblasts enhance their differentiation. Here the authors report that the isoform characterized by the presence of extradomain A (encoded by HsaEX0026101) activates alpha4beta1 integrin and augments osteoblast differentiation. In addition, the isoform containing extradomain B (encoded by HsaEX0026102) enhances the binding of fibronectin through the RGD sequence to beta3-containing integrin, resulting in increased mineralization by and differentiation of osteoblasts. These study thus reveals novel functions for two fibronectin isoforms and the mediating receptors in osteoblast differentiation.
PMID: 33207933
The authors perform quantitative proteomic analysis of the matrisome of murine carotid arteries in mice deficient in the production of FN splice isoforms containing alternative exons EIIIA and EIIIB (FN-EIIIAB null) after exposure to low and disturbed flow in vivo. They also examine serum-derived and endothelial-cell contributions to the matrisome in a simplified in vitro system. Flow-induced differences in the carotid artery matrisome that were impaired in FN-EIIIAB null mice. One of the most interesting differences was reduced recruitment of FBLN1 (fibulin-1), abundant in blood and not locally produced in the intima. This defect was validated in these in vitro assay, where FBLN1 recruitment from serum was impaired by the absence of these alternatively spliced segments.
GENOMIC CONTEXT[edit]
INCLUSION PATTERN[edit]