MmuEX0051303 @ mm10
Exon Skipping
Gene
ENSMUSG00000021614 | Vcan
Description
versican [Source:MGI Symbol;Acc:MGI:102889]
Coordinates
chr13:89685079-89712498:-
Coord C1 exon
chr13:89712205-89712498
Coord A exon
chr13:89688579-89693501
Coord C2 exon
chr13:89685079-89685192
Length
4923 bp
Sequences
Splice sites
3' ss Seq
AAACTCTTTGTTTTTTTCAGGTC
3' ss Score
11
5' ss Seq
CAGGTATGG
5' ss Score
9.99
Exon sequences
Seq C1 exon
GCGATGTGTTCCACATCACTGCTCCCAGTAAGTTCACCTTCGAGGAGGCCGAAGCAGAGTGTACAAGCAGGGATGCGAGGCTGGCGACTGTTGGAGAACTTCAGGCAGCTTGGAGAAATGGCTTTGACCAATGCGATTACGGCTGGCTGTCGGATGCCAGCGTGCGGCACCCTGTGACTGTGGCCAGGGCCCAGTGTGGAGGAGGTCTACTTGGGGTGAGAACCCTGTATCGTTTTGAGAACCAGACATGCTTCCCTCTCCCTGATAGCAGATTTGATGCCTACTGCTTTAAAC
Seq A exon
GTCGATTGAGTGATATGATTGTAAGTGGTCATCCAATAGATTCAGAATCTAAAGAAGAGGAACCTTGCAGTGAAGAAACAGATCCACTGCATGATCTGTTTGCTGAAATTTTACCCGAGTTACCAGACTCATTTGAAATAGACATATATCACAGTGAGGAAGATGAAGACGGAGAGGAGGACTGTGTAAATGCAACGGATGTAACAACTACTCCGTCAGTGCAGTATATCAATGGGAAGCAGCTCGTTACCACAGTGCCTAAGGACCCGGAAGCTGCAGAAGCTAGGCGTGGCCAGTACGAAAGTGTTGCACCTTCTCAGAATTTCCCAGATAGTTCTGCAACTGACACCCATCAGTTTATACTAGCAGAAACAGAATCGTCAACTACCATGCAATTTAAGAAATCTAAAGAAGGCACGGAATTGTTAGAAATCACATGGAAACCCGAGACCTACCCTGAAACACCAGACCATGTTTCAAGTGGTGAGCCTGATGTTTTCCCTACTCTCTCATCCCATGATGGTAAAACCACCAGATGGTCAGAGTCCATCACAGAGAGCAGTCCAAACCTTGAAAATCCAGTGCACAAACAACCTAAGCCTGTCCCTCTGTTTCCTGAAGAGTCTTCAGGAGAGGGTGCCATTGAACAAGCATCCCAGGAAACAATCTTATCTAGGGCTACAGAAGTAGCACTTGGTAAAGAGACAGACCAAAGTCCTACCTTATCTACCTCTAGCATACTTTCAAGTTCTGTGTCAGTAAATGTCTTGGAGGAAGAACCACTTACTCTAACAGGAATCTCACAGACTGATGAATCCATGTCTACTATAGAAAGCTGGGTAGAGATAACACCTAGCCAAACTGTAAAGTTCTCGGAGAGTTCTTCAGCTCCTATCATTGAAGGGTCTGGGGAAGTAGAAGAAAATAAAAATAAAATCTTTAACATGGTAACTGATTTACCACAGAGAGATCCTACAGATACACTCAGCCCTTTGGATATGAGCAAGATAATGATCACAAACCATCACATTTATATTCCTGCAACCACCGCTCCTTTGGATTCAAAACTACCTTCTCCAGATGCAAGGCCTACACAGTTTGGAATTCAAACAGCCACCTCTGAGTGGGTTTCCGATAAGTCTTTTGAAGGAAGAAAAAGAGAAGAAGATGAAGAGGGAGCTGTGAATGCAGCCCACCAAGGAGAAGTTCGAGCAGCAACAGAGAGGTCAGATCACTTACTTTTAACACCAGAATTAGAAAGTTCAAATGTAGATGCATCTAGTGATTTGGCTACCTGGGAAGGTTTTATTCTAGAGACAACACCCACAGAGTCTGAAAAGGAAATGGCAAATTCAACTCCTGTTTTTAGAGAAACAATTGGTGTAGCCAATGTGGAGGCACAGCCCTTTGAGCATAGCAGTAGTAGTCATCCCAGGGTTCAGGAAGAGCTGACCACTCTCTCAGGAAATCCCCCCTCTCTTTTTACGGACCTGGGCTCAGGAGATGCTAGTACTGGTATGGAACTCATCACTGCTTCTTTATTTACATTAGATTTAGAGTCTGAAACCAAAGTCAAAAAGGAATTACCCAGTACTCCGTCTCCCAGTGTGGAGATATCATCCTCTTTTGAGCCAACAGGATTAACCCCGAGTACTGTACTAGACATAGAAATTGCTGGAGTTATGAGCCAGACATCGCAGAAGACACTGATTTCTGAAATATCAGGAAAGCCAACTTCCCAGTCAGGAGTAAGGGATTTGTATACAGGCTTTCCAATGGGAGAAGATTTTAGTGGTGACTTCAGTGAATATCCAACAGTGTCTTATCCAACGATGAAAGAGGAAACAGTTGGGATGGGAGGTTCTGACGATGAACGAGTTAGGGATACACAGACTTCATCATCTATACCGACTACCTCAGACAACATCTACCCCGTGCCTGACTCAAAAGGACCTGACAGTACTGTGGCCAGCACCACAGCCTTCCCCTGGGAAGAGGTCATGTCCTCAGCTGAGGGGTCAGGTGAGCAATTAGCCTCAGTCAGGAGCTCTGTTGGTCCAGTGCTTCCACTAGCTGTGGACATTTTTTCTGGAACAGAGTCTCCATATTTTGATGAAGAGTTTGAAGAAGTGGCTGCAGTGACAGAAGCAAATGAAAGACCCACTGTTTTGCCAACAGCAGCAAGTGGAAATACTGTAGATCTTACAGAAAATGGGTACATAGAGGTAAATAGCACGATGTCACTGGACTTTCCCCAGACAATGGAGCCATCCAAATTGTGGTCTAAACCAGAAGTCAATCTTGATAAACAAGAGATTGGAAGAGAAACAGTGACAAAGGAAAAAGCTCAGGGACAAAAGACTTTTGAATCGCTCCACAGTTCTTTTGCACCAGAACAAACCATTTTGGAAACCCAATCACTTATTGAAACTGAATTCCAAACTTCCGATTACTCTATGTTAACAACACTGAAAACGTACATTACTAATAAGGAAGTGGAGGAGGAAGGTATGTCCATAGCGCATATGTCTACTCCAGGTCCAGGGATCAAAGACTTGGAATCATATACTACCCATCCCGAAGCTCCTGGAAAGTCACACTCTTTCTCAGCTACTGCTTTAGTGACTGAATCTGGAGCAGCTAGAAGTGTACTCATGGATTCCTCAACTCAAGAGGAAGAAAGCATAAAACTCTTCCAAAAGGGTGTGAAACTAACAAATAAAGAGTCAAATGCTGATCTCTCATTCTCTGGACTGGGATCAGGAGAGGCTTTGCCTCCTCTACCTACAACATCAGTGAATTTGACTGACATGAAGCAGATCATTAGCACATTGTATGCTGAGACATCTCACATGGAAAGCTTAGGAACAAGCATCTTAGGTGATAAAATGGAAGACCATGAAAGAATGGAAGATGTGTCCAATGAAGTTAGAATGCTCATTTCCAAAACAGGCAGCATCTCTCAAGACAGCACAGAGGCACTCGACACAACTTTGAGTCATACCGGAACAGAAGAACCTACAACTTCAACTCTTCCTTTCGTGAAGCTCATGGACTTGGAACGTTCTCCAAAGCAGACCCTCAGGTGGGAAGAGGAAACCCAGACCCATAGACCACAAACTATGAGTGGACTGATCTCTAATGAAAATTCTTCAGCATCAGAAGCTGAAGAAGCAGCAACTTCCCCCACTGCTTTTCTGCCTCAAACTTACAGTGTTGAAATGACCAAACACTTTGCTCCATCAGAGTCACAACCATCCGACTTGTTTAATGTAAACTCTGGAGAGGGATCTGGAGAAGTGGATACTCTTGACTTAGTGTACACTTCTGGAACTACTCAGGCAAGCAGTCAAGGAGACAGCATGCTTGCTTCTCATGGATTTCTGGAAAAACATCCAGAGGTGTCAAAGACTGAGGCTGGAGCTACTGATGTATCTCCAACAGCCTCAGCAATGTTCCTGCATCACTCAGAGTACAAGAGTTCTCTGTATCCAACCAGCACACTGCCAAGCACAGAGCCATATAAAAGTCCCTCAGAAGGTATTGAAGATGGTTTGCAAGACAATATCCAATTTGAAGGTTCTACCTTGAAACCTAGCAGAAGAAAAACCACTGAAAGCATTATTATAGATCTAGACAAAGAGGATAGTAAGGACCTAGGATTGACAATTACTGAGAGTGCTATTGTTGAAATTCTCCCTGAGCTGACATCAGATAAAAATATTATTATAGACATTGATCACACTAAACCTGTATATGAGTACATCCCTGGAATACAAACAGACCTAGATCCAGAGATAAAATTGGAATCACATGGCAGTAGTGAAGAAAGCCTTCAAGTTCAAGAGAAGTATGAGGGAGCTGTTACCCTTTCTCCAACTGAGGAATCATTTGAAGGCTCTGGTGATGCGCTTCTTGCTGGCTATACTCAAGCAATATACAATGAGTCTGTGACTCCCAACGATGGAAAGCAGGCAGAGGACATAAGCTTTAGTTTTGCCACTGGGATCCCAGTTTCTAGCACAGAGACAGAATTACACACTTTCTTCCCCACAGCGTCAACCCTGCACATTCCTAGTAAACTAACCACAGCTAGTCCGGAGATTGATAAACCCAACATTGAAGCAATATCTCTGGATGATATATTTGAATCAAGCACCTTGTCTGATGGCCAAGCTATTGCAGACCAGAGTGAAGTAATATCAACATTAGGGCATTTGGAAAAAACACAGGAAGAATATGAAGAAAAGAAATATGGAGGTCCTTCATTTCAGCCAGAATTCTTTTCAGGAGTGGGGGAAGTATTGACAGATCCCCCTGCTTATGTAAGTATTGGTAGTACATACCTTATAGCTCAGACTTTAACAGAATTACCCAATGTGGTAAGACCATCTGACTCCACACATTACACTGAAGCAACACCTGAAGTTTCCTCTCTGGCAGAGCTCTCTCCACAGATACCATCTTCTCCCTTCCCTGTCTACGTAGACAATGGAGTCTCTAAATTCCCAGAGGTTCCCCATACAAGTGCTCAGCCAGTTTCTACTGTCACCTCATCACAAAAGTCTATAGAGAGTCCTTTTAAGGAAGTCCATGCTAATATTGAAGAAACCATCAAGCCATTGGGTGGAAATGTCCACAGAACAGAGCCTCCATCCATGTCTCGTGACCCTGCTTTAGATGTTTCAGAAGATGAAAGTAAGCATAAGTTACTAGAAGAACTGGAAACGTCTCCCACCAAACCTGAGACTTCCCAAGACTTCCCAAACAAAGCAAAGGATCACATTCCAGGAGAAACAGTTGGGATGCTTGCTGGTATCAGAACAACAGAGAGCGAACCTGTCATTACTGCAGACGACATGGAGCTAGGAGGTGCCACACAGCAGCCACATTCTGCTTCTGCAGCTTTCAGAGTTGAGACAG
Seq C2 exon
GACCTGATCTCTGCAAAACAAACCCATGCCTCAACGGAGGCACCTGTTATCCTACCGAGACTTCCTATGTGTGCACCTGTGCACCTGGATACAGCGGAGACCAGTGTGAACTTG
VastDB Features
Vast-tools module Information
Secondary ID
ENSMUSG00000021614_MULTIEX1-2/3=C1-C2
Average complexity
C2*
Mappability confidence:
100%=100=100%
Protein Impact
Alternative protein isoforms (Ref)
No structure available
Features
Disorder rate (Iupred):
C1=0.000 A=0.750 C2=0.000
Domain overlap (PFAM):
C1:
PF0019312=Xlink=WD(100=98.0)
A:
NO
C2:
PF0000822=EGF=WD(100=79.5)
Main Inclusion Isoform:
ENSMUST00000109543fB4482
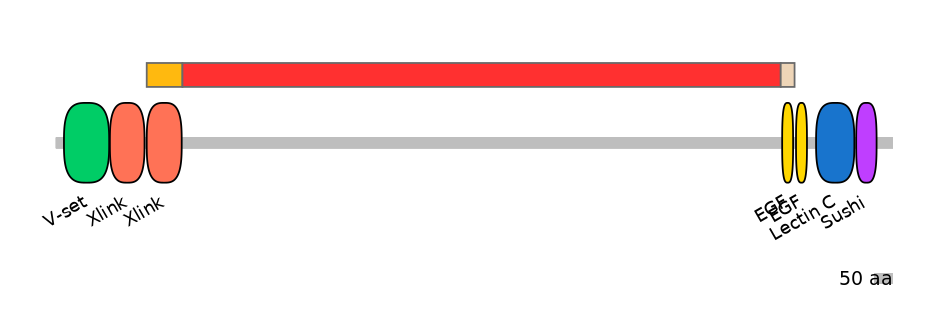
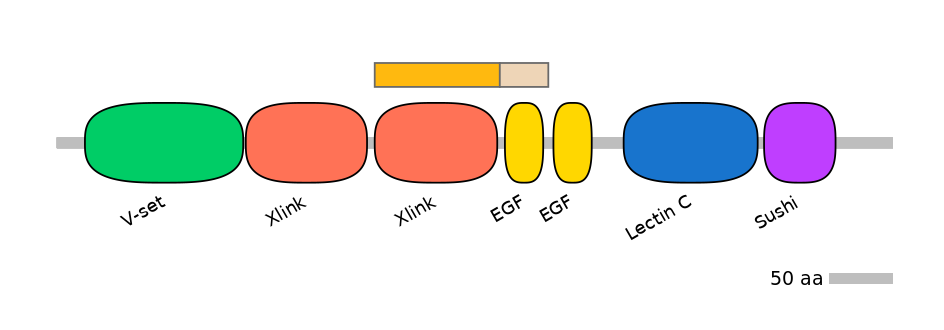
Associated events
Other assemblies
Conservation
Chicken
(galGal3)
No conservation detected
Zebrafish
(danRer10)
No conservation detected
Fruitfly
(dm6)
No conservation detected
Primers PCR
Suggestions for RT-PCR validation
F:
No suggested primer sequences
R:
No suggested primer sequences
Band lengths:
Functional annotations
There are 12 annotated functions for this event
PMID: 11807809
This event
Versican is an extracellular matrix proteoglycan produced by many cells. Although versican is generally known as a large chondroitin sulfate proteoglycan (CSPG), the smallest splice variant, V3 (lacks alpha GAG exon (HsaEX0070281) and beta GAG exon (HsaEX0070282), consists only of the amino_ and carboxy_terminal globular domains and is therefore predicted to be a small glycoprotein, lacking CS chains. To determine whether V3 alters cell phenotype, Fisher rat arterial smooth muscle cells (ASMCs), which express the larger CSPG versican splice forms (V0 (contains both GAG exons) and V1 (contains beta GAG exon and lacks alpha GAG exon) were retrovirally transduced with the rat V3 cDNA. Northern analysis for versican RNAs confirmed that cells transduced with V3 retrovirus, but not cells tranduced with the empty vector, expressed RNA of the size expected for V3/neor bicistronic RNA. V3 overexpressing cells were more spread on tissue culture plastic, had a smaller length_to_breadth ratio and were more resistant to release from the culture dish by trypsin. Interference reflection microscopy of sparsely plated cells showed larger areas of close contact between the V3 expressing cells and the coverslip, in comparison to control cells. Focal contacts in the periphery of V3 expressing cells were larger. Growth and migration studies revealed that V3 transduced cells grow slower and migrate a shorter distance in a scratch wound assay. The increased adhesion and the inhibition of migration and proliferation resulting from V3 overexpression are the opposites of the known and predicted effects of the other variants of versican. Note: in this study, the V3 is compared to an empty control. Thus, the exons are not tested in isolation.
PMID: 11884379
This event
Versican is an extracellular matrix (ECM) proteoglycan that is synthesized as multiple splice variants. V3-overexpressing ASMCs exhibit significantly increased expression of tropoelastin and increased formation of elastic fibers in long-term cell cultures. In addition, V3-overexpressing ASMCs seeded into ballooned rat carotid arteries continued to overexpress V3 and, at 4 weeks after seeding, produced a highly structured neointima significantly enriched in elastic fiber lamellae. In contrast to the hydrated, myxoid neointima produced by rounded or stellate vector-alone?transduced cells, V3-expressing cells produced a compact and highly ordered neointima, which contained elongated ASMCs that were arranged in parallel arrays and separated by densely packed collagen bundles and elastic fibers. Note, in this study, the V3 is compared to an empty control. Thus, the exons are not tested in isolation.
PMID: 16452631
This event
Versican is a large extracellular chondroitin sulfate proteoglycan that belongs to the family of lecticans. Alternative splicing of versican generates at least four isoforms named V0, V1, V2, and V3. The study shows that ectopic expression of versican V1 isoform (contains beta GAG exon (HsaEX0070281), and lacks alpha GAG exon (HsaEX0070282)) induced mesenchymal-epithelial transition (MET) in NIH3T3 fibroblasts, and inhibition of endogenous versican expression abolished the MET in metanephric mesenchyme (tested against V2, which lacks beta- GAG exon and contains alpha GAG exon). MET in NIH3T3 cells was demonstrated by morphological changes and dramatic alterations in both membrane and cytoskeleton architecture. Molecular analysis showed that V1 promoted a ?switch? in cadherin expression from N- to E-cadherin, resulting in epithelial specific adhesion junctions. V1 expression reduced vimentin levels and induced expression of occludin, an epithelial-specific marker, resulting in polarization of V1-transfected cells. Furthermore, an MSP (methylation-specific PCR) assay showed that N-cadherin expression was suppressed through methylation of its DNA promoter. Exogenous expression of N-cadherin in V1-transfected cells reversed V1's effect on cell aggregation. Reduction of E-cadherin expression by Snail transfection and siRNA targeting E-cadherin abolished V1-induced morphological alteration. Transfection of an siRNA construct targeting versican also reversed the changed morphology induced by V1 expression. Silencing of endogenous versican prevented MET of metanephric mesenchyme. Taken together, these results demonstrate the involvement of versican in MET: expression of versican is sufficient to induce MET in NIH3T3 fibroblasts and reduction of versican expression decreased MET in metanephric mesenchyme.
PMID: 16510447
This event
It was previously shown that the selective expression of the chondroitin sulfate proteoglycans versican V0 (contains both alpha- and beta GAG exons) and V1 (contains beta GAG exon (HsaEX0070282) and lacks alpha GAG exon (HsaEX0070281)) in barrier tissues that impede the migration of neural crest cells during embryonic trunk development (Landolt et al, Devel 1995). To test for an active involvement of these isoforms in the guidance process, the study established protocols to isolate intact versican V0 and V1 in quantities sufficient for functional experiments. Using stripe choice assays, the authors demonstrate that pure preparations of either a mixture of versican V0/V1 or V1 alone strongly inhibit the migration of multipotent Sox10/p75NTR double-positive early neural crest stem cells on fibronectin by interfering with cell-substrate adhesion. This inhibition is largely core glycoprotein-dependent, as the complete removal of the glycosaminoglycan chains has only a minor effect on the inhibitory capacity.
PMID: 19712046
This event
A promising method to fabricate tissue-engineered blood vessels is to have cells synthesize the supportive extracellular matrix scaffold of the tissue-engineered blood vessel; however, a shortcoming of this method has been limited elastogenesis. Arterial smooth muscle cells (ASMCs) produce significant quantities of elastin when transduced with splice variant 3 of the proteoglycan versican (V3) (lacks alpha- (HsaEX0070281) and beta (HsaEX0070282) GAG exons). This study assessed whether elastogenesis and the structural properties of entirely cell-derived engineered vascular constructs could be improved by the incorporation of V3-transduced rat ASMCs. After 18 weeks of culture, V3 constructs had more tropoelastin, more elastin crosslinks, higher burst strengths, greater elasticity, and thicker collagen fiber bundles compared with empty-vector controls. The expression of elastin and elastin-associated proteins was increased in V3 and control ASMC monolayer cultures when ascorbic acid, which promotes collagen synthesis and inhibits elastogenesis, was removed from the medium. Engineered vascular constructs with ascorbate withdrawn for 14 weeks, after an initial 4-week exposure to ascorbate, exhibited increased elastin, desmosine content, elasticity, and burst strength compared with constructs exposed continuously to ascorbate. These results show that V3 coupled with limited exposure to ascorbate promotes elastogenesis and improves the structural and functional properties of engineered vascular constructs. Note: in this study, the V3 is compared to an empty control. Thus, the exons are not tested in isolation.
PMID: 10671370
Versican V2 strongly inhibits neurite outgrowth of central and peripheral neurons in stripe-choice assays using laminin-1 as permissive substrate.
PMID: 15635104
Overexpression of V1 (contain CS (chondroitin sulfate) beta => only HsaEX0070281) enhances cell growth, prevents apoptosis, and activates the ERK pathway. V2 (contain CS (chondroitin sulfate) alpha => only HsaEX0070282) overexpression inhibits cell growth, the ERK pathway, and axon regeneration after injury. V1 knockdown decreases ERK phosphorylation and increases sensitivity to HA14-mediated apoptosis.
PMID: 14695326
Transduction of skin fibroblasts from Costello syndrome and Hurler disease patients with cDNA to versican V3 (skipping of both exons 7 and 8) completely reverses impaired elastogenesis and restores normal proliferation of these cells. This phenotypic reversal is accompanied by loss of chondroitin sulfate from the cell surface and increased levels of EBP. Versican V3 transduction of skin fibroblasts from GM(1)-gangliosidosis patients, which lack EBP, failed to restore impaired elastogenesis.
PMID: 14978219
Expression of versican V1 isoform (MmuEX0051302) in PC12 cells induced complete differentiation, whereas expression of V2 (MmuEX0051303) induced an aborted differentiation accompanied by apoptosis. V1 promoted neurite outgrowth of hippocampal neurons, but V2 failed to do so.
PMID: 16847433
The presence of versican in the extracellular matrix plays a role in tumor cell growth, adhesion and migration, which could be altered by altering the ratio between versican isoforms. Overexpression of the V3 isoform of versican in human melanoma cell lines markedly reduces cell growth in vitro and in vivo, since V3-overexpressing (LV3SN) (VCAN lacking both alpha- (HsaEX0070281) and beta (HsaEX0070282) GAG exon) cultured cells as well as primary tumors arising from these cells grow slower than their vector-only counterparts (LXSN). This study demonstrates that the delayed cell growth is due to multiple events since differences in proliferative index as well as in apoptosis are observed in LV3SN cells and tumors compared to LXSN. LV3SN melanoma cells exhibit delayed activation of MAPK in response to EGF, the authors have also characterized further the primary tumors originated in nude mice from V3-transduced melanoma cells to determine if other events affect the V3 tumor phenotype. Hyaluronan content of LV3SN tumors was higher than in LXSN tumors, whereas other related matrix components and vascularization were unaffected. Furthermore, lung metastasis in nude mice occurred only in animals carrying LV3SN tumors, indicating a dual role for this molecule, both as an inhibitor of tumor growth and a metastasis inductor.
PMID: 21671478
To clarify the roles of versican in chondrosarcoma, versican splicing variant 1 (contains beta GAG exon (HsaEX0070282) and lacks alpha GAG exon (HsaEX0070281)), variant 3 (lacks both alpha- and beta GAG exons) or only GFP was stably transfected to Swarm rat chondrosarcoma cells with Trap-In System. Forced expression of versican V1 isoform in Swarm rat chondrosarcoma cells induced a marked increase of cell-associated matrix compared to V3-, GFP- transfected or RCS cells. Versican was immunolocalized in a fashion similar to that of hyaluronan and more diffusively than aggrecan. Anchor-dependent and -independent growth was not affected by versican isoform expression, whereas cell motility and migration were significantly enhanced by V1 isoform transfection. Tumors formed in vivo with V1-transfected cells exhibited more myxomatous area and included more spindle shaped cells.
PMID: 30394708
The endometrium extracellular matrix (ECM) is essential for embryo implantation. Versican, a large chondroitin sulfate proteoglycan that binds hyaluronan and forms large ECM aggregates, can influence fundamental physiological phenomena, such as cell proliferation, adhesion and migration. The present study investigated the possible role of versican in human embryo implantation. Versican V1 (contains beta GAG exon (HsaEX007082) and lacks alpha GAG exon (HsaEX007081)) expression and secretion in human endometrial epithelial cells (EECs) was most prominent in the mid-secretory phase. Versican expression in EECs significantly increased after treatment with estrogen and progesterone, but not by estrogen alone. They also established versican V1-overexpressing Ishikawa (endometrial cancer cell line) cells (ISKW-V1), versican V3-overexpressing (ISKW-V3) (V3 lacks both alpha- and beta GAG exons) and control GFP-overexpressing (ISKW-GFP) Ishikawa cells. By the in vitro implantation model, the attachment ratio of BeWo (choriocarcinoma cell line) spheroids to the monolayer of ISKW-V1, but not of ISKW-V3, was found significantly enhanced compared with attachment to the ISKW-GFP monolayer. The conditioned medium derived from ISKW-V1 (V1-CM) also promoted the attachment of BeWo spheroids to the ISKW monolayer. However, this attachment-promoting effect was abolished when V1-CM was pretreated with chondroitinase ABC, which degrades chondroitin sulfate. Therefore, out of the ECM components, versican V1 may facilitate human embryo implantation.
GENOMIC CONTEXT[edit]
INCLUSION PATTERN[edit]
SPECIAL DATASETS
- Pre-implantation embryo development
- Ribosome-engaged transcriptomes of neuronal types
- Neural differentiation time course
- Muscular differentiation time course
- Spermatogenesis cell types
- Reprogramming of fibroblasts to iPSCs
- Hematopoietic precursors and cell types