RnoEX0040005 @ rn6
Exon Skipping
Gene
ENSRNOG00000011726 | Grin1
Description
glutamate ionotropic receptor NMDA type subunit 1 [Source:RGD Symbol;Acc:2736]
Coordinates
chr3:2508411-2512421:-
Coord C1 exon
chr3:2512276-2512421
Coord A exon
chr3:2510913-2511023
Coord C2 exon
chr3:2508411-2509000
Length
111 bp
Sequences
Splice sites
3' ss Seq
CTTATACATATTCATTTTAGGAT
3' ss Score
8.75
5' ss Seq
ACGGTAAGG
5' ss Score
10.38
Exon sequences
Seq C1 exon
GGGTCTTCATGCTGGTGGCTGGAGGCATCGTAGCTGGGATTTTCCTCATTTTCATTGAGATCGCCTACAAGCGACACAAGGATGCCCGTAGGAAGCAGATGCAGCTGGCTTTTGCAGCCGTGAACGTGTGGAGGAAGAACCTGCAG
Seq A exon
GATAGAAAGAGTGGTAGAGCAGAGCCCGACCCTAAAAAGAAAGCCACATTTAGGGCTATCACCTCCACCCTGGCCTCCAGCTTCAAGAGACGTAGGTCCTCCAAAGACACG
Seq C2 exon
AGCACCGGGGGTGGACGCGGCGCTTTGCAAAACCAAAAAGACACAGTGCTGCCGCGACGCGCTATTGAGAGGGAGGAGGGCCAGCTGCAGCTGTGTTCCCGTCATAGGGAGAGCTGAGACGCCCCGCCCGCCCTCCTCTGCCCCTCCCCCGCAGACAGACGCACGGGACAGCGGCCTGGCCCACGCAGAGCCCCGGAGCACGACGGGGTCGGGGGAGGAGCACTCCCAGCCTCCCCCAGGCCGTGCCCGCCTGCCCACCGGTCGGCCGGCTGGCCGGTCCACCCTGTCCCGGCCCCGCGCGTGCCCCCGACGTCGGAGCTAACGGGCCGCCTTGTCTGTGTATTTCTATTTTACAGCAGTACCATCCCACTGATATCACGGGCCCGCTCAACCTCTCAGATCCCTCGGTCAGCACCGTGGTGTGAGGCCCCCCGGAGGCGCCCACCTGCCCAGTTAGCCCGGCCAAGGACACTGATGAGTCCTGCTGCTCGGGAAGGCCT
VastDB Features
Vast-tools module Information
Secondary ID
ENSRNOG00000011726_CASSETTE1
Average complexity
S
Mappability confidence:
100%=100=100%
Protein Impact
Alternative protein isoforms (Ref)
No structure available
Features
Disorder rate (Iupred):
C1=0.007 A=0.635 C2=0.579
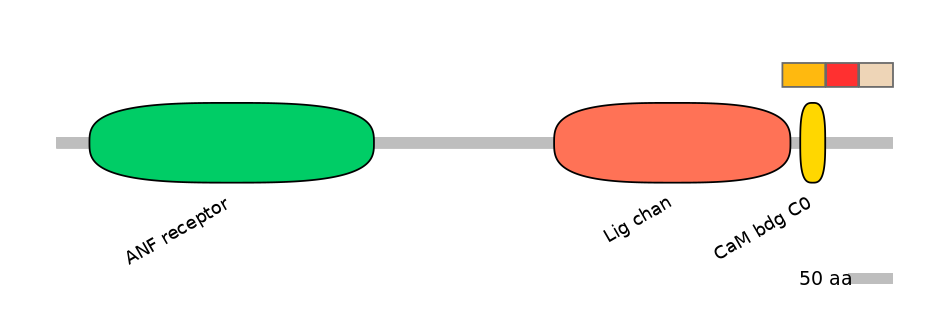
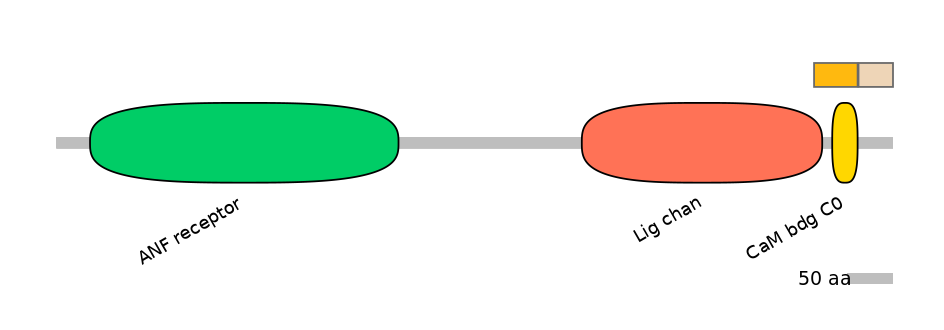
Domain overlap (PFAM):
C1:
PF0006021=Lig_chan=PD(3.4=18.4),PF105624=CaM_bdg_C0=WD(100=59.2)
A:
NO
C2:
NO
Other Skipping Isoforms:
NA
Associated events
Conservation
Fruitfly
(dm6)
No conservation detected
Primers PCR
Suggestions for RT-PCR validation
F:
GAGGCATCGTAGCTGGGATTT
R:
GCGTCTCAGCTCTCCCTATGA
Band lengths:
247-358
Functional annotations
There are 6 annotated functions for this event
PMID: 8515865
In the presence of calcium, the reduction of total current was greatest in the NMDAR1-LL splice variant, and was significantly less in the NMDAR1-SS variant. The increased sensitivity of NMDAR1-LL may be attributed to a particularly sensitive slow current 'hump' which is more pronounced in NMDAR1-LL than in NMDAR1-SS. The ethanol sensitivity of these receptorsranged in order as NR1-1b (LL) > NR1-2b (LS) > NR1-1a (SL) > NR1-2a (SS), although this subunit dependence was lost whenrecordings were carried out in barium-containing media. LL = insertion of both HsaEX0028686 and HsaEX0028687. SS = skipping of both.
PMID: 9030583
Encodes an experimentally validated Eukaryotic Linear Motif (ELM). Method: mutation analysis, polyclonal antibody, western blot. ELM ID: ELMI002147; ELM sequence: RRSSKDT; Overlap: FULL
PMID: 11312291
Encodes an experimentally validated Eukaryotic Linear Motif (ELM). Method: colocalization, confocal microscopy, mutation analysis, mutation disrupting interaction, sequence based prediction. ELM ID: ELMI002064; ELM sequence: RRR; Overlap: FULL
PMID: 16573586
In this study, all 8 NR1 splice variants were individually coexpressed with each NR2 subunit in human embryonic kidney 293 (HEK293) cells and tested for inhibition by ethanol using patch-clamp electrophysiology. All 32 subunit combinations tested gave reproducible glutamate-activated currents and all receptors were inhibited to some degree by 100 mM ethanol. The sensitivity of individual receptors to ethanol was affected by the specific NR1 splice variant expressed with receptors containing the NR1-3 and NR1-4 subunits among the least inhibited by ethanol. The isoforms NR1-3 and NR1-4 could not be mapped (no info in the paper).
PMID: 10479681
When NR1 splice variants were expressed in fibroblasts, the amount of NR1 molecules expressed on the cell surface varied among forms with different C-terminal cytoplasmic domains. The splice forms with the longest C-terminal cytoplasmic tail (NR1-1a and NR1-1b) showed the lowest amount of cell surface expression, and the splice forms with the shortest C-terminal cytoplasmic tail (NR1-4a and NR1-4b) showed the highest cell surface expression. Cells transfected with 1b, 2b, 3b, or 4b demonstrate different levels of [Ca2+] increase following glutamate stimulation. N-cassette: MmuEX0021891??, C1: MmuEX0021891, C2-C2': MmuALTA0008176.
PMID: 34187890
The GluN1 subunit contains eight alternatively spliced isoforms produced by including or excluding the N1 and the C1, C2, or C2' polypeptide cassettes. Whether GluN1 alternative splicing affects nonionotropic signaling by NMDARs is a major outstanding question. Here, the authors discovered that glycine priming of recombinant NMDARs critically depends on GluN1 isoforms lacking the N1 cassette; glycine priming is blocked in splice variants containing N1. On the other hand, the C-terminal cassettes-C1, C2, or C2'-each permit glycine signaling. In wild-type mice, the authors found glycine-induced nonionotropic signaling at synaptic NMDARs in CA1 hippocampal pyramidal neurons. This nonionotropic signaling by glycine to synaptic NMDARs was prevented in mice the authors engineered, such that GluN1 obligatorily contained N1. The authors discovered in wild-type mice that, in contrast to pyramidal neurons, synaptic NMDARs in CA1 inhibitory interneurons were resistant to glycine priming. But the authors recapitulated glycine priming in inhibitory interneurons in mice engineered such that GluN1 obligatorily lacked the N1 cassette. Ex 5: MmuEX0021893, C-term cassettes: MmuALTA0008176 and MmuEX0021891.
GENOMIC CONTEXT[edit]
INCLUSION PATTERN[edit]