GgaEX0006465 @ galGal3
Exon Skipping
Gene
ENSGALG00000004719 | SPTAN1
Description
NA
Coordinates
chr17:5806400-5809543:+
Coord C1 exon
chr17:5806400-5806417
Coord A exon
chr17:5808263-5808325
Coord C2 exon
chr17:5809489-5809543
Length
63 bp
Sequences
Splice sites
3' ss Seq
GGTTTCTTTTCTTCGATTAGCAT
3' ss Score
4.5
5' ss Seq
AAGGTTTTT
5' ss Score
3.43
Exon sequences
Seq C1 exon
GACTTACCTGCTAGATGG
Seq A exon
CATAGCCTATCGTCGTGTCATTCGTGTCTATCAGTATGAAGTTGGGGATGATCTATCTGGAAG
Seq C2 exon
GTCCTGCATGGTGGAGGAGTCGGGAACACTGGAATCCCAGCTGGAAGCTACTAAA
VastDB Features
Vast-tools module Information
Secondary ID
ENSGALG00000004719_MULTIEX2-33/40=32-34
Average complexity
C1
Mappability confidence:
NA
Protein Impact
Alternative protein isoforms (No Ref)
No structure available
Features
Disorder rate (Iupred):
C1=0.000 A=0.000 C2=0.505
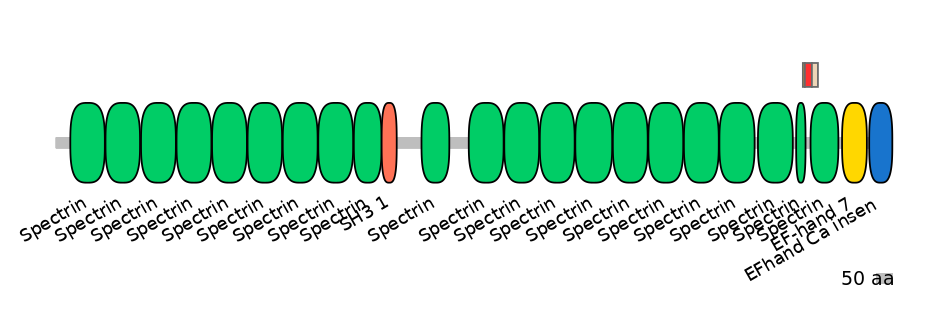
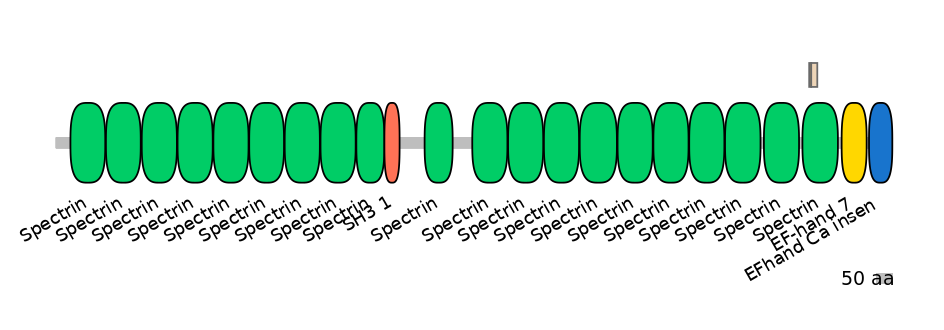
Domain overlap (PFAM):
C1:
PF0043516=Spectrin=FE(20.7=100)
A:
PF0043516=Spectrin=PD(6.9=9.1),PF0043516=Spectrin=PU(4.8=18.2)
C2:
PF0043516=Spectrin=FE(16.8=100)
Other Inclusion Isoforms:
NA
Associated events
Other assemblies
Conservation
Fruitfly
(dm6)
No conservation detected
Primers PCR
Suggestions for RT-PCR validation
F:
No suggested primer sequences
R:
No suggested primer sequences
Band lengths:
Functional annotations
There are 1 annotated functions for this event
PMID: 20114050
In vitro studies of recombinant alphaII-spectrin polypeptides representing the two splice variants of alphaII-spectrin, alphaII-cardi+ (with MmuEX0044726) and alphaII-cardi_ (without MmuEX0044726), revealed that the alphaII-cardi+ subunit has lower affinity for the complementary site in repeats 1-4 of betaII-spectrin, with a KD value of ~1 nM, as measured by surface plasmon resonance (SPR). In addition, the alphaII-cardi+ form showed 1.8-fold lower levels of binding to its site on betaII-spectrin than the alphaII-cardi_ form, both by SPR and blot overlay. This suggests that the 21-amino acid insert prevented some of the alphaII-cardi+ form from interacting with betaII-spectrin. Fusion proteins expressing the alphaII-cardi+ sequence within the two terminal spectrin repeats of alphaII-spectrin were insoluble in solution and aggregated in neonatal myocytes, consistent with the possibility that this insert removes a significant portion of the protein from the population that can bind beta subunits. Neonatal rat cardiomyocytes infected with adenovirus encoding GFP-fusion proteins of repeats 18-21 of alphaII-spectrin with the cardi+ insert formed many new processes. These processes were only rarely seen in myocytes expressing the fusion protein lacking the insert or in controls expressing only GFP. These results suggest that the embryonic mammalian heart expresses a significant amount of alphaII-spectrin with a reduced avidity for beta-spectrin and the ability to promote myocyte growth.
GENOMIC CONTEXT[edit]
INCLUSION PATTERN[edit]