MmuEX0041272 @ mm10
Exon Skipping
Gene
ENSMUSG00000032511 | Scn5a
Description
sodium channel, voltage-gated, type V, alpha [Source:MGI Symbol;Acc:MGI:98251]
Coordinates
chr9:119539524-119550734:-
Coord C1 exon
chr9:119550606-119550734
Coord A exon
chr9:119543324-119543415
Coord C2 exon
chr9:119539524-119539754
Length
92 bp
Sequences
Splice sites
3' ss Seq
ACTGTGGTTTGGTCCCACAGGTA
3' ss Score
10.09
5' ss Seq
CAGGTAAGG
5' ss Score
11.08
Exon sequences
Seq C1 exon
GTACACCTTCACCGCCATCTACACCTTTGAGTCTCTGGTCAAGATTCTAGCTCGAGGCTTCTGCCTGCATGCGTTCACCTTCCTCCGGGACCCGTGGAACTGGCTAGACTTCAGTGTGATCGTCATGGC
Seq A exon
GTATGTATCAGAGAATATAAAGCTAGGCAATTTGTCGGCTCTTCGAACTTTCAGAGTCCTGAGAGCTCTGAAAACGATTTCAGTTATTCCAG
Seq C2 exon
GCCTGAAGACAATCGTGGGAGCCCTAATCCAGTCTGTGAAGAAGCTAGCCGATGTGATGGTCCTTACTGTCTTCTGCCTCAGTGTCTTTGCCCTCATTGGCCTGCAGCTCTTCATGGGCAACCTGAGGCACAAGTGCGTGCGTAACTTTACTGAGCTCAATGGCACCAATGGTTCCGTGGAGGCCGATGGCATAGTCTGGAACTCTCTGGACGTCTACCTCAATGACCCAG
VastDB Features
Vast-tools module Information
Secondary ID
ENSMUSG00000032511_MULTIEX1-1/2=C1-C2
Average complexity
ME(1-2[99=100])
Mappability confidence:
100%=100=100%
Protein Impact
ORF disruption upon sequence exclusion
No structure available
Features
Disorder rate (Iupred):
C1=0.000 A=0.000 C2=0.000
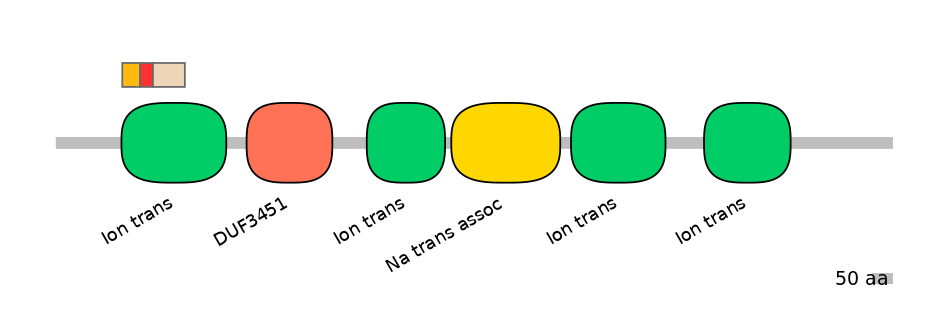
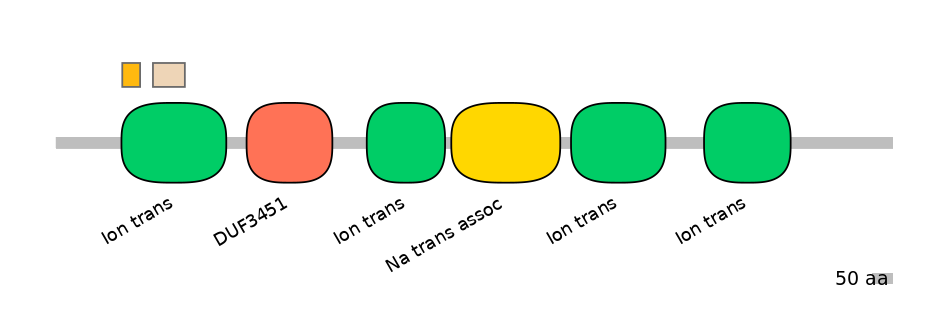
Domain overlap (PFAM):
C1:
PF0052026=Ion_trans=FE(16.9=100)
A:
PF0052026=Ion_trans=FE(12.2=100)
C2:
PF0052026=Ion_trans=FE(30.3=100)
Other Inclusion Isoforms:
NA
Associated events
Other assemblies
Conservation
Chicken
(galGal3)
No conservation detected
Fruitfly
(dm6)
No conservation detected
Primers PCR
Suggestions for RT-PCR validation
F:
CACCTTCACCGCCATCTACAC
R:
CGGCTAGCTTCTTCACAGACTG
Band lengths:
177-269
Functional annotations
There are 5 annotated functions for this event
PMID: 27063795
This event
Using RNA sequencing, this study identifies novel splicing alterations in DM heart samples, including a switch from adult exon 6B (HsaEX0056559) towards fetal exon 6A (HsaEX0056558) in the cardiac sodium channel, SCN5A. MBNL1 regulates alternative splicing of SCN5A mRNA and that the splicing variant of SCN5A produced in DM presents a reduced excitability compared with the control adult isoform. Importantly, reproducing splicing alteration of Scn5a in mice is sufficient to promote heart arrhythmia and cardiac-conduction delay, two predominant features of myotonic dystrophy
PMID: 30371314
This event
The sodium channel, Nav1.5, encoded by SCN 5A, undergoes developmentally regulated splicing from inclusion of exon 6A in the fetal heart (MmuEX0041272) to exon 6B (MmuEX0041273) in adults. These mutually exclusive exons differ in 7 amino acids altering the electrophysiological properties of the Nav1.5 channel. In myotonic dystrophy type 1, SCN 5A is mis-spliced such that the fetal pattern of exon 6A inclusion is detected in adult hearts. Cardiac manifestations of myotonic dystrophy type 1 include conduction defects and arrhythmias and are the second-leading cause of death. This work aimed to determine the impact of SCN5A mis-splicing on cardiac function. The authors used CRISPR-Cas9 to delete Scn5a exon 6B in mice, thereby redirecting splicing toward exon 6A. These mice exhibit prolonged PR and QRS intervals, slowed conduction velocity, extended action potential duration, and are highly susceptible to arrhythmias. These findings highlight a nonmutational pathological mechanism of arrhythmias and conduction defects as a result of mis-splicing of the predominant cardiac sodium channel. Animals homozygous for the deleted exon express only the fetal isoform and have more-severe phenotypes than heterozygotes that also express the adult isoform.
PMID: 18393272
HsaEX0056558 and HsaEX056559 are mutually exclusive and are included in neonatal and adult tissue, respectively. They encode exon 6a (fetal) and 6b (adult). Exon 6a/6b encodes amino acid located in D1:S3-S3-S4 linker. The two exons differ at 7 amino acid positions. Electrophysiological characterization: 'neonatal' and 'adult' Nav1.5 alpha-subunit splice variants were stably transfected into EBNA-293 cells and their electrophysiological properties investigated by whole-cell patch-clamp recording. Compared with the 'adult' isoform, the 'neonatal' channel exhibited (1) a depolarized threshold of activation and voltage at which the current peaked; (2) much slower kinetics of activation and inactivation; (3) 50% greater transient charge (Na(+)) influx; (4) a stronger voltage dependence of time to peak; and (5) a slower recovery from inactivation. Tetrodotoxin sensitivity and VGSCbeta1-4 mRNA expression levels did not change. The significance of the charge-reversing aspartate to lysine substitution was investigated by mutating the lysine in the 'neonatal' channel back to aspartate. In this 'neonatal K211D' mutant, the electrophysiological parameters studied strongly shifted back towards the 'adult', that is the lysine residue was primarily responsible for the electrophysiological effects of Nav1.5 D1:S3 splicing.
PMID: 21552533
Nine Nav1.5 (SCN5A) splice variants are known. Four of them, namely Nav1.5a, Nav1.5c, Nav1.5d, and Nav1.5e, generate functional channels in heterologous expression systems. In the present study, the authors systematically investigated electrophysiological properties of mutant T1620K channels in the background of all known functional Nav1.5 splice variants in HEK293 cells. This mutation is associated with two cardiac excitation disorders: long QT syndrome type 3 (LQT3) and isolated cardiac conduction disease (CCD). When investigating the effect of the T1620K mutation, the authors noticed similar channel defects in the background of hNav1.5, hNav1.5a, and hNav1.5c. In contrast, the hNav1.5d background produced differential effects: In the mutant channel, some gain-of-function features did not emerge, whereas loss-of-function became more pronounced. In case of hNav1.5e, the neonatal variant of hNav1.5, both the splice variant itself as well as the corresponding mutant channel showed electrophysiological properties that were distinct from the wild-type and mutant reference channels, hNav1.5 and T1620K, respectively. In conclusion, these data show that alternative splicing is a mechanism capable of generating a variety of functionally distinct wild-type and mutant hNav1.5 channels. Thus, the cellular splicing machinery is a potential player affecting genotype-phenotype correlations in SCN5A channelopathies. (About variants: Variant a and c differs from the canonical variant by skipping exon 18 (HsaEX0056556), and including Q1077 (HsaALTA0007521-2/2), respectively. Negative results for those. Variant D seems to use alternative splice sites in exon 17 (not in VastDB), and variant E uses the neonatal version of the ME exon (HsaEX0056558), while the other variants use the adult version (HsaEX0056559)).
PMID: 22064211
Congenital long-QT syndrome (LQTS) may present during fetal development and can be life-threatening. The molecular mechanism for the unusual early onset of LQTS during fetal development is unknown. Genetic analysis disclosed a novel, de novo heterozygous mutation (L409P) in the cardiac sodium channel (SCN5A) and a homozygous common variant (R558 in SCN5A). In vitro electrophysiological studies demonstrated that the mutation in combination with R558 caused significant depolarized shifts in the voltage dependence of inactivation and activation, faster recovery from inactivation, and a 7-fold higher level of persistent current. When the mutation was engineered in a fetal-expressed SCN5A splice isoform (uses the mutually exclusive exon HsaEX0056558), channel dysfunction was markedly potentiated (compared to the adult isoform, which uses the mutually exclusive exon HsaEX0056559). Also, R558 alone in the fetal splice isoform evoked a large persistent current, and hence both alleles were dysfunctional.
GENOMIC CONTEXT[edit]
INCLUSION PATTERN[edit]
SPECIAL DATASETS
- Pre-implantation embryo development
- Ribosome-engaged transcriptomes of neuronal types
- Neural differentiation time course
- Muscular differentiation time course
- Spermatogenesis cell types
- Reprogramming of fibroblasts to iPSCs
- Hematopoietic precursors and cell types